- Title: Associated diseases
- Subtitle: Melisa
- Intro text editor:
People with metal hypersensitivity may have numerous symptoms associated with an overactive immune system, including chronic fatigue, joint and muscle pain, cognitive impairment, depression, headaches, fibromyalgia and skin rashes.
The article A comprehensive summary of disease variants implicated in metal allergy lists hundreds of publications describing metal-specific allergy responses, identifying over 50 unique manifestations. According to the authors, metals constitute one of the major classes of allergens responsible for a disproportionately large segment of the total burden of disease associated with allergy. Excerpts are available HERE.
Another recent review article looks at the connection between metal hypersensitivity and implant failure. You can check the full article here: The contribution of metal allergy to the failure of metal alloy implants, with special reference to titanium: Current knowledge and controversies. A brief excerpt focused on several case reports of clinical reactions to implants is available HERE.
- List item:
- List title:
Increased reactivity to metals has been found in the following diseases:, List text:
• Multiple Sclerosis (Prochazkova 2003, 2006, Stejskal 2006)
• Chronic Fatigue Syndrome (Stejskal 1994, 1999)
• Rheumatoid Arthritis (Prochazkova 2003, Stejskal 2006)
• Fibromyalgia (Öckert 2006, Stejskal 2013)
• Amyotrophic Lateral Sclerosis (Pleva 2000)
• Cardiovascular disease (Manousek 2016)
• Lupus Erythematosis (Prochazkova 2003)
• Oral Lichen Planus (Stejskal 1996)
• Oral burning and itching (Stejskal 2006)
• Skin diseases such as eczema or psoriasis (Prochazkova 2003, Venclikova 2003, Kohdera, Ionescu)
• Sjögren’s syndrome (Prochazkova 2003)
• Alopecia areata and atopic alopecia (Nakayama and Chen 2018)
• Autoimmune thyroiditis (Sterzl 1999, Prochazkova 2003, 2010, Hybenova 2010)
The prevalence of metal hypersensitivity in patients with implants is significantly higher than in the general population, with an even higher rate among patients with failed or failing implanted devices. (Hallab et al. Metal sensitivity in patients with orthopedic implants. The Journal of Bone and Joint Surgery 2001;83:428)
We are conducting a study to see if metals may be implicated in the development of scoliosis. Read more about the research HERE. - List title:
Treatment of diseases where metal allergy has played a part, List text:
As with any allergy, exposure to the offending allergen should be minimized or, if possible, avoided altogether.
Metals can be found in food and also in medication, such as nickel and titanium dioxide, so in this case, patients might consider diets low in nickel. If a patient is allergic to a metal found in dental fillings or dental implants, it is important to consult a dentist who is experienced in the field of metal-free dentistry. The importance of being protected during the removal of, for example, amalgam fillings, cannot be overstated. Every dentist will have their own protocols, for instance, the SMART protocol from the International Academy of Oral Medicine and Toxicology (IAOMT). For many patients, avoiding metals will be enough for them to feel a significant health improvement.
- List title:
Increased reactivity to metals has been found in the following diseases:, List text:
- Title: Implant hypersensitivity
- Subtitle: metal exposure
- Intro text editor:
The MELISA test may be used in two ways for orthopaedic patients. First, prior to surgery, patients whose clinical history suggests metal sensitivity may be pre-tested to ensure that they receive the most suitable implant, more info HERE. Second, post-surgery, MELISA can be used to identify if metal hypersensitivity is responsible for any of the symptoms that have developed.
Patients suffering from metal hypersensitivity may have numerous local symptoms associated with an overactive immune system, such as localised pain, swelling, cutaneous allergic reactions, joint and muscle pain, implant failure, apparent recurrent infections around the operation site, and possible systemic reactions such as fibromyalgia, chronic fatigue and cognitive impairment. MELISA is a scientifically proven and clinically validated blood test that detects type-IV hypersensitivity to multiple metals at the same time.
- List item:
- List title:
Metal hypersensitivity and orthopaedics, List text:
Metal hypersensitivity is a well-documented factor in the failure of implants, and the need for testing in sensitive patients is well recognized by both implant manufacturers and by surgeons alike. The prevalence of metal hypersensitivity in patients with implants is significantly higher than in the general population, with an even higher rate among patients with failed or failing implanted devices.
- List title:
Implant alloys, List text:
An exact breakdown, which includes trace amounts of metals present, is needed prior to testing, but below is a guideline. Metals usually found in common medical grade alloys include:
Cobalt chrome: cobalt, chromium, manganese, molybdenum, nickel, tungsten
Stainless steel: chromium, manganese, molybdenum, nickel, tungsten
Titanium alloy (TiAl6V4): titanium, vanadium, aluminium, traces of nickelRecently, cases of titanium hypersensitivity have also been described. Titanium is a transition metal and thus may function as a hapten and trigger cellular hypersensitivity. Since titanium, in the form of titanium dioxide (E171), is used as white pigment in toothpaste, cosmetics and medication, the latent sensitization of susceptible individuals is possible. Traces of nickel (0.03%) may be found even in commercially pure titanium due to the production process.
- List title:
Stents, clips and coils, List text:
Both bare metal and drug eluting stents have been occasionally implicated in the developments of various hypersensitivity reactions. More information about the metals commonly contained in stents can be found HERE. It is important to establish which metals are contained in your device prior to testing.
- List title:
Metal hypersensitivity and orthopaedics, List text:
- References:
References
Valentine-Thon E, Schiwara HW. Validity of MELISA® for metal sensitivity testing. Neuroendocrinology Letters 2003; 24(1/2):57-64
Aesculap Implant systems brochure 2009 www.aesculapimplantsystems.com
Rushinga G, Goretsky M, Gustin T, Morales M, Kelly R, Nuss D. When it is not an infection: metal allergy after the Nuss procedure for repair of pectus excavatum. Journal of Pediatric Surgery 2007;42:93-97
Adala R, Chakravarthy M, Srinivas V, Pai S. Orthopaedic surgery in a patient with metal sensitivity. J Cutan Aesthet Surg. 2011 Jan-Apr; 4(1): 67-68.
Hallab, N. PhD, Merritt, K. PhD, Jacobs, J. Metal sensitivity in patients with orthopedic implants. The Journal of Bone and Joint Surgery 2001;83:428.
Cohen D. How safe are metal-on-metal hip implants? BMJ 2012;344:e1410
Grammatopolous G, Pandit H, Kwon YM, Gundle R, McLardy-Smith P, Beard DJ, et al. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg Br 2009;91:1019-24.
Stejskal V, et al. Metal-specific lymphocytes: biomarkers of sensitivity in man. Neuroendocrinology Letters 1999;20:289-298
Prochazkova J, Sterzl I, Kucerova H, Bartova J, Stejskal V. The beneficial effect of amalgam replacement on health in patients with autoimmunity. Neuroendocrinology Letters 2004;25(3):211-218.
Thomas P, Bandl WD, Maier S, Summer B, Przybilla B. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006.Oct;55(4):199-202.
Evrard L, Waroquier D, Parent D. Allergies to dental metals. Titanium: a new allergen. Rev Med Brux. 2010 Jan-Feb; 31(1):44-9.
Schuh A, Thomas P, Kachler W, Goske J, Wagner L, Holzwarth U, Forst R. Allergic potential of titanium implants. Orthopade. 2005. Apr;34(4):327-8, 330-3.
Niki Y, Matsumoto H, Otani T, Yatabe T, Kondo M, Yoshimine F, Toyama Y. Screening for symptomatic metal sensitivity: a prospective study of 92 patients undergoing total knee arthroplasty. Biomaterials. 2005. Mar;26(9):1019-26.
Schalock P, Menne T, Johansen J, Taylor J, Maibach H, Liden C, Bruze M, Thyssen J. Hypersensitivity reactions to metallic implants – diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis. 2011 July, 66, 4-19.
Basko-Pilluska J, Thyssen J, Schalock P. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011 Mar-Apr;22(2):65-79.
Syburra T, Schurr U, Rahn M, Graves K, Genoni M. Gold-coated pacemaker implantation after allergic reactions to pacemaker compounds. Europace. 2010 May;12(5):749-50.
Dörner T, Haas J, Loddenkemper C, von Baehr V, Salama A. Implant-related inflammatory arthritis. Rheumatology. 2006. 2, 53-56.
Jacobs JJ, Hallab NJ. Loosening and osteolysis associated with metal-on-metal bearings: A local effect of metal hypersensitivity?J Bone Joint Surg Am. 2006 Jun;88(6):1171-2.
Hallab NJ, Anderson S, Stafford T, Glant T, Jacobs JJ. Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. 2005. Mar;23(2):384-91.
Hallab NJ, Anderson S, Caicedo M, Skipor A, Campbell P, Jacobs J. Immune responses correlate with serum-metal in metal-on-metal hip arthroplasty. J Arthroplasty. 2004;19(8 Suppl 3):88-93.
Ross IB, Warrington RJ, Halliday WC. Cell-mediated allergy to a cerebral aneurysm clip: case report. Neurosurgery. 1998 Nov;43(5):1209-11.
- Title: Beryllium
- Subtitle: Metal exposure
- List item:
- List title:
Where is Beryllium found?, List text:
Beryllium is a metal that is used in the manufacturing of products like cars, golf clubs and computers, manufacture of thermal coating, nuclear reactors, rocket heat shields, brakes, x-ray tubes, and dental plates. It is found in some dental crowns and dental plates, but exposure to the dust is highest during the manufacturer so those working in dental laboratories may be exposed.
- List title:
What is beryllium disease?, List text:
Beryllium-induced lung disease can occur when beryllium dust or fumes are inhaled. Chronic beryllium disease (CBD, berylliosis) is associated with inhaling beryllium powder or fumes (although inhaling beryllium does not always lead to CBD). An exposed person usually gets sensitized to beryllium prior to progressing to CBD. Sensitization is similar to an allergy; when allergic or sensitized, the body reacts negatively to that particular substance. Beryllium sensitivity (BeS) and CBD can develop soon after exposure or many (30-40) years later. Of those working around beryllium, about 10% get sensitized to it and about half of those progress to develop CBD.
According to NIOSH (the National Institute for Occupational Safety and Health, 2011), “workers exposed to particles, fumes, mists and solutions from beryllium-containing materials may develop beryllium sensitization or chronic beryllium disease, a potentially disabling or even fatal respiratory disease.” Depending on how workers are exposed, the diseases can affect different tissues and organs. Breathing in fumes or dusts of beryllium compounds may injure the lungs. While most commonly associated with diseases of the lungs, beryllium may also affect such organs as the liver, kidneys, heart, nervous system, and the lymphatic system.
The Centers for Disease Control and Prevention (CDC) states that “sensitization has been found in one to ten percent of workers in cross-sectional studies, with chronic beryllium disease diagnosed in ten to 100 percent of the sensitized”. This statement means that between 1 to 10% of workers who work with beryllium may become sensitized. Of those workers who become sensitized, 10% or all may later develop chronic beryllium disease. Another study stated that on average, 1 to 6% of exposed workers may develop sensitivity, but it may be as high as 16% in workplaces with high exposure levels.
- List title:
How can it be diagnosed?, List text:
A blood test called beryllium lymphocyte proliferation test (BeLPT) can measure how blood cells react to beryllium. This test can be used for medical surveillance programs. NIOSH states that “it is believed that a person must first be sensitized before beryllium in the lungs can cause the lung damage (called granulomas) of chronic beryllium disease. However, the overall proportion of all sensitized individuals who will eventually develop chronic beryllium disease is not known.”
MELISA is involved with a working group that aims to bring standardised beryllium sensitivity testing to Europe to allow exposed workers to be tested for hypersensitivity.
Links for the specification for testing for Beryllium hypersensitivity can be found HERE.
- List title:
Where is Beryllium found?, List text:
- References:
Read more:
Cleveland Clinic
Canadian Centre for Occupational Health and Safety
- Title: Mercury allergy
- Subtitle: Metal exposure
- Intro text editor:
There are different forms of mercury, MELISA testing can differentiate between allergy to each form. Each type has its different properties and is therefore utilized in different ways in dentistry, medicine and industry or is present in the environment. Allergy to mercury is specific, so an individual can be allergic to inorganic mercury and not to phenylmercury. This is because the cells involved in Type IV allergic reaction – memory T cells – will recognise a specific form of mercury. Naturally, it is possible that an individual can be allergic to all four types of mercury. In many cases it is important to locate the source of exposure to mercury that causes inflammation in the body, so that exposure can be stopped.
- List item:
- List text:
Inorganic mercury, or ‘metallic mercury’, is a frequent source of metal allergy. It constitutes 50% of dental amalgam fillings. Dental authorities accept that mercury vapour constantly evaporates from the fillings, but argue this is below a safe limit. However, for hypersensitive patients, there is no safe limit.
In 2020 The FDA issued the following statement: “The FDA has found that certain groups may be at greater risk for potential harmful health effects of mercury vapor released from the device. As a result, the agency is recommending certain high-risk groups avoid getting dental amalgam whenever possible and appropriate.” For more information, please check HERE.
One of the groups at greater risk for potential harmful health effects is “People with known heightened sensitivity (allergy) to mercury or other components of dental amalgam.”
Replacing amalgam fillings with non-metallic alternatives has delivered radical health improvements in patients who tested MELISA-positive for mercury. 71% of patients showed health improvements in this study.
- List text:
Methylmercury is the most toxic form of mercury. It affects the immune system, alters genetic and enzyme systems, and damages the nervous system, including coordination and the senses of touch, taste, and sight. Methylmercury is particularly damaging to developing embryos, which are five to ten times more sensitive than adults. Methylmercury becomes concentrated as it move up the food chain, so the large predator fish such as shark and swordfish have the highest concentrations. Bacteria in the body can transform inorganic mercury into methylmercury.
- List text:
Phenylmercury is the organic mercury most commonly found in dental root fillings. While it has been phased out in many countries, it is also used as a preservative in eye drops and nose drops. Phenylmercury is used to control the growth of fungus in some interior latex paints manufactured before 1991, some exterior and oil base paints, some caulks, eye-area cosmetics, toiletries and other products.
- List text:
Ethylmercury is a form of organic mercury. It forms a part of thimerosal, which is used as a preservative in some vaccines, eye drops and nasal sprays.
- List text:
Thimerosal is one of the most controversial substances is modern medicine. Its main component is ethyl mercury (49.6% by weight), yet it is still used as a preservative in some childhood vaccines, most flu vaccines and some eye drops and contact lens solution. It is being withdrawn from vaccines in many countries.
- List title:
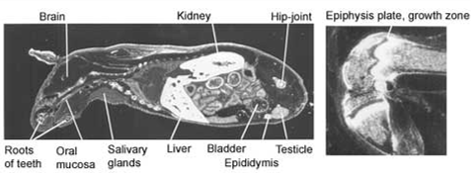
Full body autoradiograph, List text:
A special form of mercury can be used to demonstrate that mercury binds to the body proteins. The distribution of mercury in various organs of the body can be traced by sensitive photographic emulsion. Below are four pictures of mice that were injected with mercury labelled with a radioactive isotope. Then, using autoradiography, a special picture was produced. The areas where the mercury is deposited are shown in white.
The pictures demonstrate widespread distribution of mercury in the body of the mice. Organs rich in fat – such as brain and collagen – are very prone to mercury binding. One of the reasons for this is that mercury is particularly keen to bind to two amino acids; methionine and cysteine. Both amino acids contain sulphur hydrogen (SH)-groups. This is a particularly attractive target for mercury. Fat tissues and collagen tissues are rich in SH-groups.
- List text:
- References:
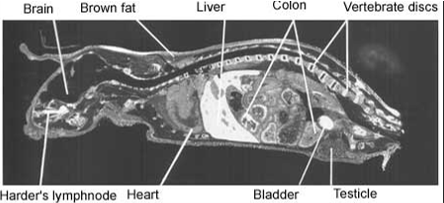
Figure 1. Distribution of radioactivity in male mouse 6 hours after intravenous injection of 203HgCl2. Magnification 2x.
Figure 2. Distribution of radioactivity in male mouse 24 hours after intravenous injection of 203HgCl2. Magnification 2x.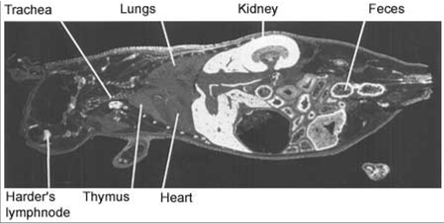
Figure 3 and 4. Distribution of radioactivity in male mouse 2 days after intravenous injection of 203HgCl2. Magnification 2x and 9x (right).